Breast PET-CT Imaging and Biopsy System
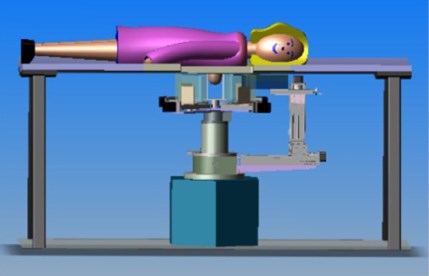
PEM/PET-Breast Imager
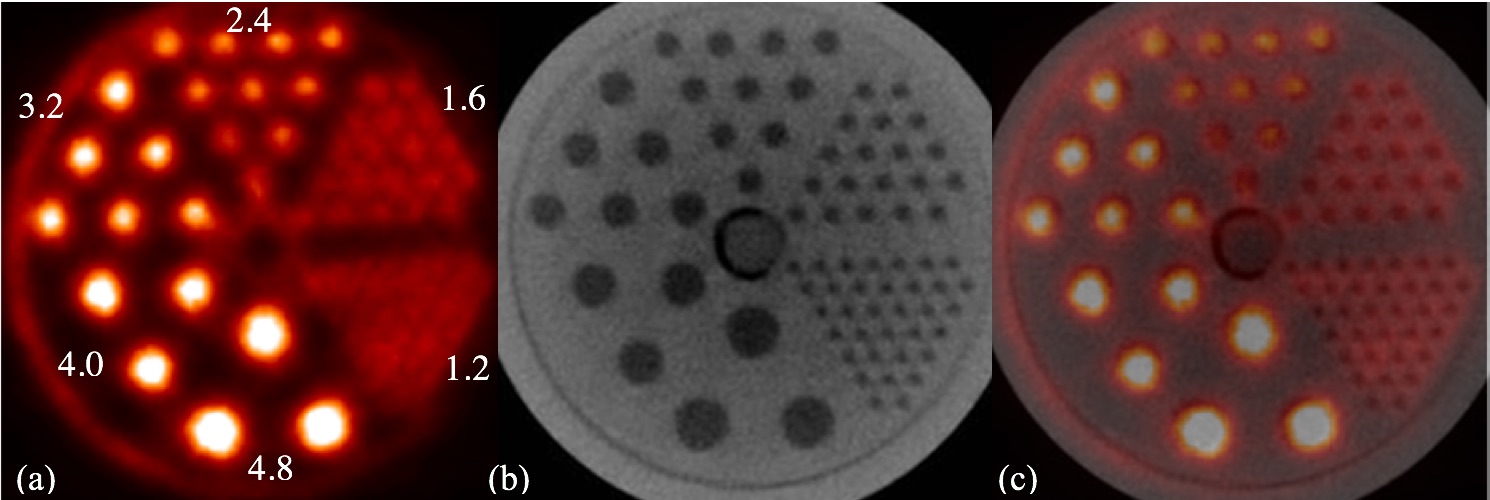
Tomographic breast imaging techniques can potentially improve detection and diagnosis of cancer in women with radiodense and/or fibrocystic breasts. We have developed a high-resolution positron emission mammography/tomography imaging and biopsy device (called PEM/PET) to detect and guide the biopsy of suspicious breast lesions. PET images are acquired to detect suspicious focal uptake of the radiotracer and guide biopsy of the area. Limited-angle PEM images could then to verify biopsy needle position prior to tissue sampling. The PEM/PET scanner consists of two sets of rotating planar detector heads. Each detector consists of a 4x3 array of Hamamatsu H8500 flat panel position sensitive photomultipliers (PSPMTs) coupled to a 96×72 array of 2×2×15mm3 LYSO detector elements (pitch= 2.1mm). Image reconstruction is performed with a three-dimensional, ordered set expectation maximization (OSEM) algorithm parallelized to run on a multi-processor computer system. The reconstructed field-of-view (FOV) is 15×15x15cm3. Initial phantom-based testing of the device is focusing upon its PET imaging capabilities. Specifically, spatial resolution and detection sensitivity were assessed. The results from these measurements yielded a spatial resolution at the center of the FOV of 2.01±0.09mm (radial), 2.04±0.08mm (tangential) and 1.84±0.07mm (axial). At a radius of 7cm from the center of the scanner, the results were 2.11±0.08mm (radial), 2.16±0.07mm (tangential) and 1.87±0.08mm (axial). Maximum system detection sensitivity of the scanner is 488.9kcps/mCi/ml (6.88%).
This project is a collaborative effort amongst West Virginia University, the University of Maryland Medical school, the University of Washington and Jefferson Lab.

Patient Images
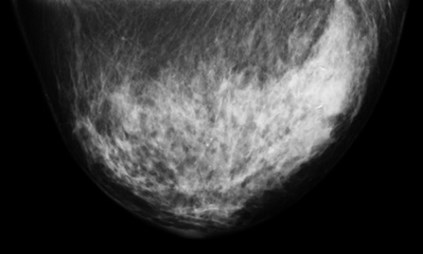

Breast-PET-Guided Biopsy
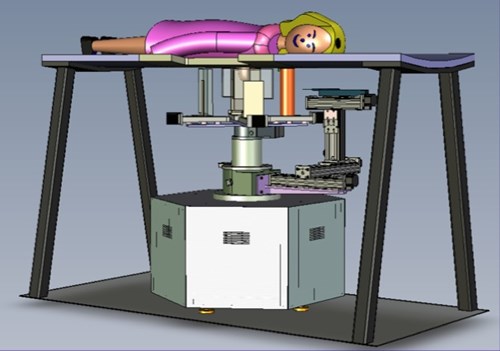
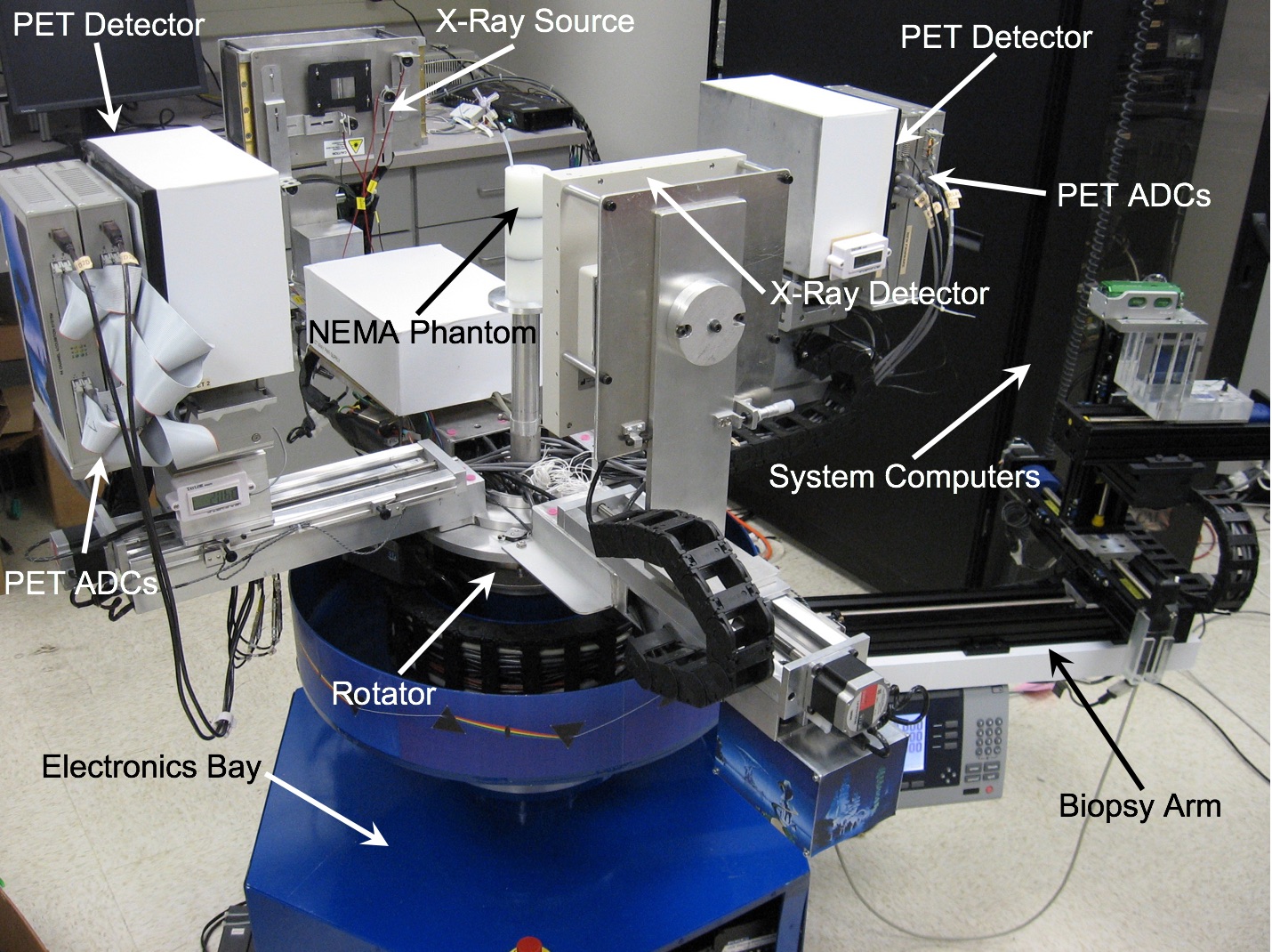
In addition to imaging the breast, the PEM/PET system is also capable of acquiring samples from objects seen in images such as those shown above. The biopsy component of the system consists of an apparatus that can translate in all three dimensions, as well as rotate 360° about the imaging axis of the scanner. Figure 1 shows the PEM-PET imaging and biopsy system. Three computer-controlled linear sliders (Velmex, Inc.) are aligned along the x, y and z axes of the biopsy arm. These sliders are mounted on a fixture that is connected via a thrust bearing to the imaging gantry. Thus, the arm can be rotated manually about the patient’s breast to select the best entry angle. The system interface software continually monitors this angle. A standard Bard Magnum® biopsy gun (C.R. Bard, Inc.) can be mounted to the arm using a specially designed fixture.
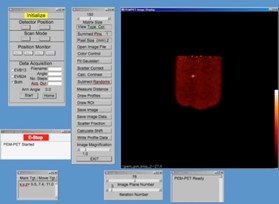
The PEM/PET imaging and biopsy system user interface is written in the Interactive Display Language (IDL). This interface interacts with Java clients that control data acquisition, image reconstruction, image display and biopsy needle targeting. To calculate the three-dimensional position of an area identified in an image, the user places the computer’s cursor on the region and clicks a mouse button. The position of the lesion in the scanner’s coordinate system is then calculated. By clicking another button, the software converts the calculated position to the number of steps necessary to position the needle to the proper location and then moves it in the x-y plane (plane parallel to the detector faces). The required depth of the needle (z-axis position) is shown on the user interface screen, but no movement in this axis is initiated. Needle motion in the z-axis is controlled via a handheld joystick unit. Thus, insertion into the patient is under the control of the user. The position of the needle is continually monitored via linear position encoders on all three axes of the biopsy arm. The outputs from these devices are displayed on a video screen located in the equipment bay of the system so that the user can keep track of the needle as it is inserted into the breast.

Dedicated Breast-PET/CT Scanner
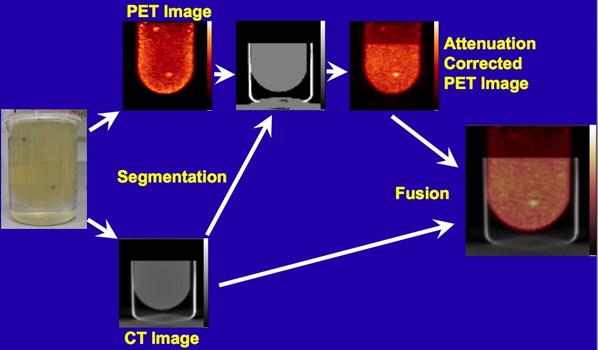
Following the successful development and testing of the PEM/PET scanner, creation of the second generation of this scanner was initiated. Specifically, the Nuclear Medicine Instrumentation Research group collaborated with Xoran, LLC to add a CT imaging capability. Additionally, the performance of the PET component was enhanced by the improving of the scintillation light optics of the detectors to improve delineation of detector elements (improving spatial resolution). Furthermore, the count rate capabilities of the system was increased by segmenting the event triggering electronics and developing a new, high speed coincidence unit in collaboration with Mesytec, GmBH.
Acknowledgements
This work was supported in part by the National Cancer Institute (Grant Number R01 CA094196).
Breast-PET Publications from our Group
- R.R Raylman, S. Majewski, R. Wojcik, A.G. Weisenberger, B. Kross, V. Popov, H.A. Bishop. The Potential Role of Positron Emission Mammography (PEM) in the Detection of Breast Cancer: A Phantom Study, Medical Physics, 2000;27(8):1943-1954.
- R.R. Raylman, S. Majewski, A.G. Weisenberger, V. Popov, R. Wojcik, B. Kross, J.S. Schreiman, H. A. Bishop. Positron Emission Mammography-Guided Breast Biopsy, Journal of Nuclear Medicine, 2001;42:960-966.
- R.R. Raylman, S. Majewski, R. Wojcik, A.G. Weisenberger, B. Kross, V. Popov. Corrections for the Effects of Accidental Coincidences, Compton Scatter and Object Size In Positron Emission Mammography (PEM) Imaging, IEEE Transactions on Nuclear Science, 2001;48:913-923.
- R.R. Raylman, S. Majewski, M.F. Smith, R. Wojcik, A.G. Weisenberger, B. Kross, V. Popov, J.J. Derakhshan, Comparison of Scintillators for Positron Emission Mammography (PEM) Systems, IEEE Transactions on Nuclear Science, 2003;50:42-49.
- M.F. Smith, S. Majewski, A.G. Weisenberger, D.A. Kieper, R.R. Raylman, T.G. Turkington, Analysis of Factors Affecting Positron Emission Mammography (PEM) Image Formation, IEEE Transactions on Nuclear Science, 2003;50:53-59.
- R.R. Raylman, M.F. Smith, P. R. Menge, A Monte-Carlo Simulation Study of Detector Array Design for Breast metabolic Imaging Systems, Nuclear Instruments and Methods in Physics Research, Section A, 2005;555:403-410.
- R.R. Raylman, S. Majewski, B. Kross, V. Popov, J. Proffitt, M.F. Smith, A.G. Weisenberger, R. Wojcik, Development of a Dedicated Positron Emission Tomography System for the Detection and Biopsy of Breast Cancer, Nuclear Instruments and Methods in Physics Research, Section A, 2006;564(2):291-295.
- R.R. Raylman, S. Majewski, M.R. Mayhugh, Light Sharing in Multi-Flat-Panel-PMT PEM Detectors, Physica Medica, 2007;XXI (suppl. 1):83-86.
- R.R. Raylman, M.F. Smith, A Task-based Evaluation of PEM Detector Element Size, Physica Medica, 2007;XXI (suppl. 1):80-82.
- R.R. Raylman, S. Majewski, M.F. Smith, J Proffitt, W. Hammond, A. Srinivasan, J. KcKisson, V. Popov, A. Weisenberger, C.O. Judy, B. Kross, S. Ramasubramanian, L.E. Banta, P.E. Kinahan, K. Champley, The Positron Emission Mammography/Tomography Breast Imaging and Biopsy System (PEM/PET): Design, Construction and Phantom-Based Measurements, Physics in Medicine and Biology, 2008;53:637-653.
- K. Champley, M. Defrise, R. Clackdoyle, R.R. Raylman, P.E. Kinahan, Planogram Rebinning with the Frequency-Distance Relationship, IEEE Transactions on Medical Imaging, 2008;27(7);925-933.
- R.R. Raylman, Positron Emission Tomography-Guided Biopsy with a Dedicated Breast Scanner: Initial Experience, IEEE Transactions on Nuclear Science, 2009;56(3):620-624.
- Champley, R. R. Raylman, P.E. Kinahan, Advancements to the planogram frequency-distance rebinning algorithm, Inverse Problems, 2010;26:10-38. (NIHMSID 191512)
- R.R. Raylman, J. Abraham, H. Hazard,C. Koren, S. Filburn, J.S. Schreiman, S. Kurian, S. Majewski, G.D. Marano, Initial Clinical Test of a Breast-PET Scanner, Journal of Medical Imaging and Radiation Oncology, 2011;55(1):58-64. (NIHMSID 285474).
- R.R. Raylman, K. Vaigneur, A.V. Stolin, G. Jaliparthi, G, Arrays of Segmented, Tapered Light Guides for Use With Large, Planar Scintillation Detectors, IEEE Transactions on Nuclear Science, 2015;62(3):694-698 (NIHMSID 712407).
- R. Raylman, W. Van Kampen, A.V. Stolin, W. Gong, G. Jaliparthi, P.F. Martone, M.F. Smith, D. Sarment, N.H. Clinthorne, M. Perna, A dedicated breast-PET/CT scanner: Evaluation of basic performance characteristics. Medical Physics, 2018;45(4):1603-1613. (PMID: 29389017)